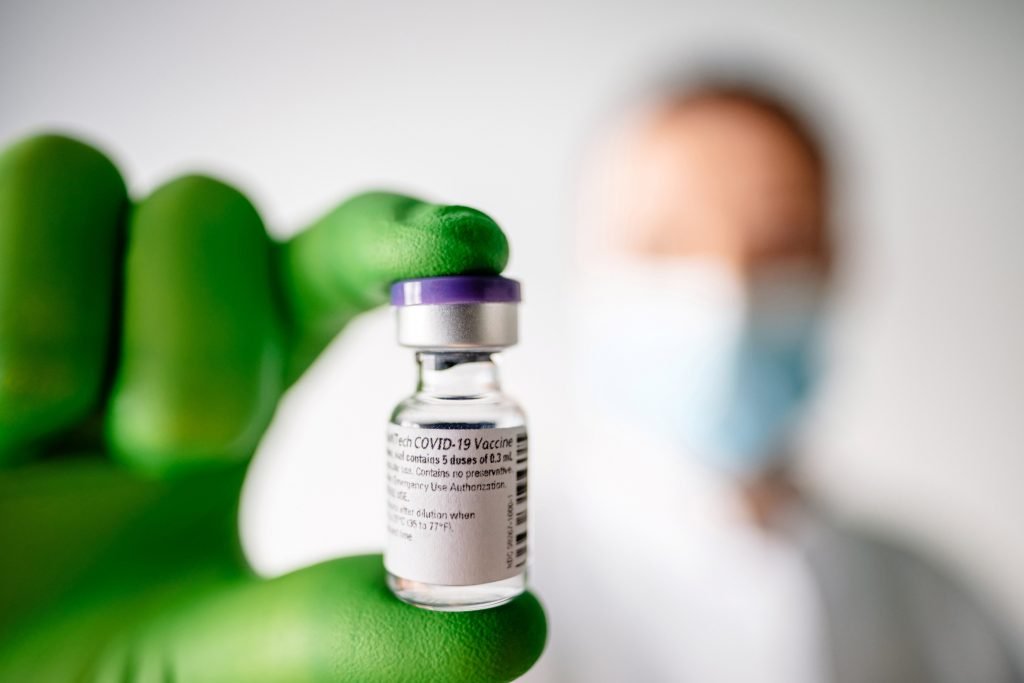
First Vaccine Approved by the Saudi Food and Drug Authority. Corresponding to December 10, 2020 AD, that it has approved the registration of the “Pfizer-BioNTech” Coronavirus ( Pfizer-BioNTech COVID-19 Vaccine ) in the Kingdom of Saudi Arabia after the company “Pfizer” submitted to request approval to register it so that the health authorities in the Kingdom can then import the vaccine and use it.
First Vaccine Approved by the Saudi Food and Drug Authority
The decision to approve the registration of the vaccine and to allow its use came based on the data provided by the company, “Pfizer” on November 24, 2020 AD. As soon as the requirements are completed, the authority began the process of reviewing and evaluating the registration files from several aspects, including the evaluation of the vaccine efficacy and safety data shown by it. Clinical trials and studies, as well as verifying the quality of the vaccine by reviewing scientific data that shows the quality of manufacturing and the stability of the product, in addition to verifying the manufacturing stages and the manufacturer’s commitment to applying the principles of good pharmaceutical manufacturing ( GMP ) according to international standards in the pharmaceutical industry.
The authority held several meetings to study the data provided by the company, which included meetings with local and international experts and scientists, in addition to meeting with the manufacturer and its representatives to answer the inquiries submitted by the authority, and the opinion of the scientific advisory team for infectious diseases emanating from the Scientific Advisory Committee for Clinical Studies was also taken.
Continue
According to the system of pharmaceutical and herbal facilities and preparations, the committee for registering pharmaceutical companies and factories and their products held a meeting to study the data and scientific reports, and after presenting and discussing the issue in all its technical and scientific aspects, the committee decided to approve the registration of the vaccine and allow its use.
On the date of arrival of the vaccine and starting to use it, the authority clarified that based on the approval issued today, the concerned health authorities will start import procedures according to the standards and requirements for this, and the authority will analyze samples from each incoming shipment of the vaccine before using it to ensure its quality, and the Ministry of Health will announce the date of the arrival of the vaccine And start using it after completing import requirements.
More from Riyadh Xpress
Elephant Mountain | Al Ula | Saudi Arabia
Al Ula | Fort | Old Town | Discover Saudi Arabia
Stay Safe by following precautionary measures given by the government
- Firstly, free movement with taking care of precautionary measures given by the government. Cautiously we returned to normal life.
- Secondly, staying at home can be very boring, we have made a list of things to do here.
- Thirdly, wear a face mask all the time if you are in a gathering or outside.
- Fourthly, wash your hands regularly for a minimum of 20 seconds.
- Lastly, buy a protected mask: Click Here.
Finally, follow Riyadh Xpress’s:
In addition, check out the latest articles by Riyadh Xpress
- Firstly, King Salman urged the world to respond to the human crises causes by Covid-19: Click Here.
- Secondly, Social Distancing Measures implemented in supermarkets: Click Here.
- Thirdly, the latest penalties on Iqama: Click Here.
- Fourthly, Disabled parking violation/fine in Saudi Arabia: Click Here.
- Lastly, hackers attack again to get your personal data: Click Here.
Check out the most viewed articles
- Firstly, one riyal shop in Riyadh: Click Here.
- Secondly, 5 Riyals Mall in Riyadh: Click Here.
- Thirdly, Riyal al Barakah: Click Here.
- Lastly, 20 new changes in Saudi Arabia in 2020: Click Here.